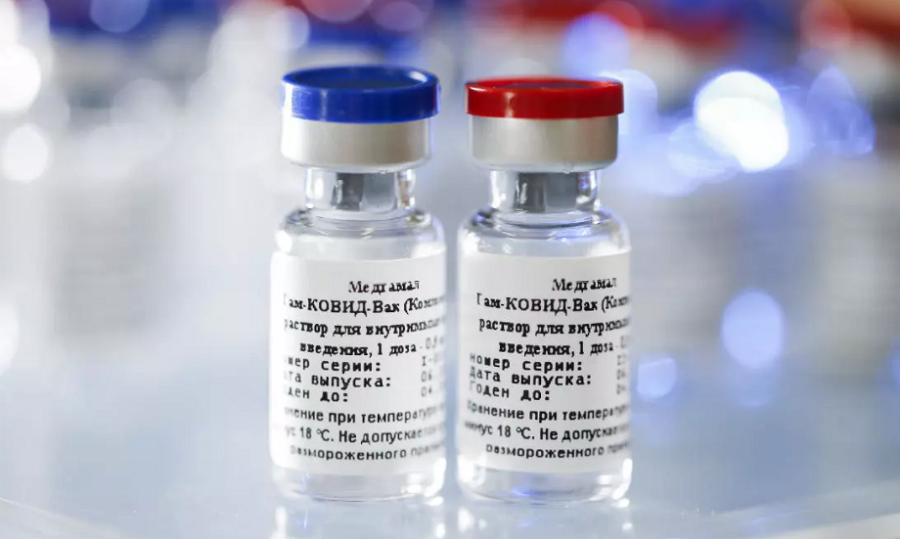
Russia has announced that it has approved its third vaccine against the coronavirus disease for domestic use.
Moscow was the first country to register a vaccine against Covid-19, Sputnik V, in August 2020, ahead of trials.
According to a report from Reuters, this was disclosed by the Russian Prime Minister, Mikhail Mishustin, while speaking on State TV, on Saturday, although large-scale clinical trials of the vaccine, labelled CoviVac and produced by the Chumakov Centre, is yet to commence.
Mishustin said, “Today, Russia is the only country to have already three vaccines against COVID-19.’’
READ: Macron proposes 4-5% of COVID-19 vaccine doses for poorer nations
Russia has already approved 2 Covid-19 vaccines, including the Sputnik V shot, developed by Moscow’s Gamaleya Institute and has been approved in over 2 dozen countries across the globe.
The preemptive approvals which were first greeted with scepticism, had some scientists from the West raise concerns, but vaccinations with those first two shots began on a mass scale in Russia only after trials were concluded and showed success.
Sputnik V was approved in August and late-stage trials began in September. Mass vaccination was launched in December after preliminary trial results showed the vaccine to be 91.4% effective.
READ: Covid-19: Prime Minister of Eswatini dies of the virus
The Russian Health Minister, Mikhail Murashko had revealed that over 2 million Russians have been vaccinated with at least the first dose of Sputnik V, since then.
The rollout of a second vaccine, developed by the Vector Institute in Novosibirsk, is beginning.
The CoviVac vaccine which was produced by state-run Chumak Centre based in Moscow has employed a different method from Sputnik and EpiVacCorona, using inactive virus.
Unlike the Sputnik V vaccine, which uses a modified harmless cold virus that tricks the body into producing antigens to help the immune system prepare for a coronavirus infection, the CoviVac vaccine is a whole-virion vaccine.
READ: COVID-19: WHO advises vaccine firms and wealthy nations to stop bilateral deals
The Chumakov Centre’s director, Aidar Ishmukhametov, said on Saturday, “The vaccine we have developed… reflects the whole history of Russian, as well as global, vaccine science.’’
He said that the Chumakov Centre is expecting to produce around half a million doses per month on its platforms, Ishmukhametov said on Saturday.
Deputy Prime Minister Golikova also announced on Saturday that Russia will produce 88 million vaccine doses in the first half of this year, including 83 million Sputnik V doses.
What you should know
- It can be recalled that in a televised government meeting, the Russian President, Vladimir Putin, had announced the registration of its first Covid-19 vaccine in what was then seen as a step ahead of other vaccine development.
- The vaccine which was developed by Russia’s Gamaleya Institute in collaboration with the Russian Investment Fund was said to give effective protection against the deadly disease.
- Russian President Vladimir Putin announced in October that the country had registered its second vaccine, EpiVacCorona, which health officials had said would enter mass production this month.