The National Agency for Food and Drugs Administration and Control (NAFDAC) has alerted healthcare providers regarding performance concerns with certain lots of Abbott’s m-PIMA HIV-1/2 VL cartridges.
Abbott has identified that these specific lots do not meet the performance claims for HIV-1 group O and HIV-1 group M and N throughout the claimed shelf life, based on findings from an internal stability monitoring program. It’s important to note that the performance of HIV-2 is not affected.
Abbott is actively investigating the root cause of this issue and has committed to taking necessary corrective actions to prevent any recurrence.
The impacted lots were identified through a rigorous statistical analysis.
The m-PIMA HIV-1/2 VL cartridge is a point-of-care nucleic acid test that directly quantifies HIV type 1 groups M/N and O, as well as HIV type 2 RNA in human plasma samples. It differentiates HIV-1 and HIV-2 using Polymerase Chain Reaction (PCR) technology.
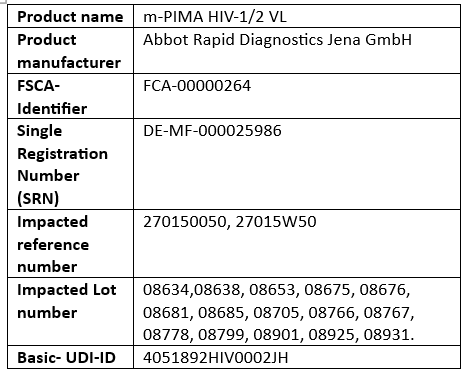
Product details

Potential risks
There is a potential risk of falsely low viral load level results for HIV-1 group O and HIV-1 group M and N patients, which could lead to delayed follow-up, inappropriate treatment adjustments, progression of infection, and deterioration of patient health.
Actions expected from healthcare providers and patients
Healthcare providers and patients should inspect the current inventory on hand to determine if they have the impacted lots. Immediate discontinuation of use and quarantine of any remaining inventory is advised.
Healthcare providers should review previously reported patient results using the impacted lots and, if deemed appropriate, notify patients for retesting.
Reporting Guidelines
Healthcare professionals and consumers are urged to report any suspicions of adverse reactions, substandard or falsified medicines, diagnostic devices, and other regulated products to the nearest NAFDAC office.
Reports can be made through the toll-free number 0800-162-3322 or via email at sf.alert@nafdac.gov.ng.
Additionally, healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of NAFDAC-regulated products.
Reports can be submitted to the nearest NAFDAC office or through the E-reporting platforms on the NAFDAC website (www.nafdac.gov.ng) or the Med-safety application available for download on Android and IOS stores. Reports can also be sent via email to pharmacovigilance@nafdac.gov.ng.
NAFDAC remains committed to ensuring the safety and efficacy of healthcare products in Nigeria, and this advisory aims to keep healthcare providers and patients informed about potential risks associated with the use of specific Abbott m-PIMA HIV-1/2 VL cartridges.






















Thanks for your enlighten me and others