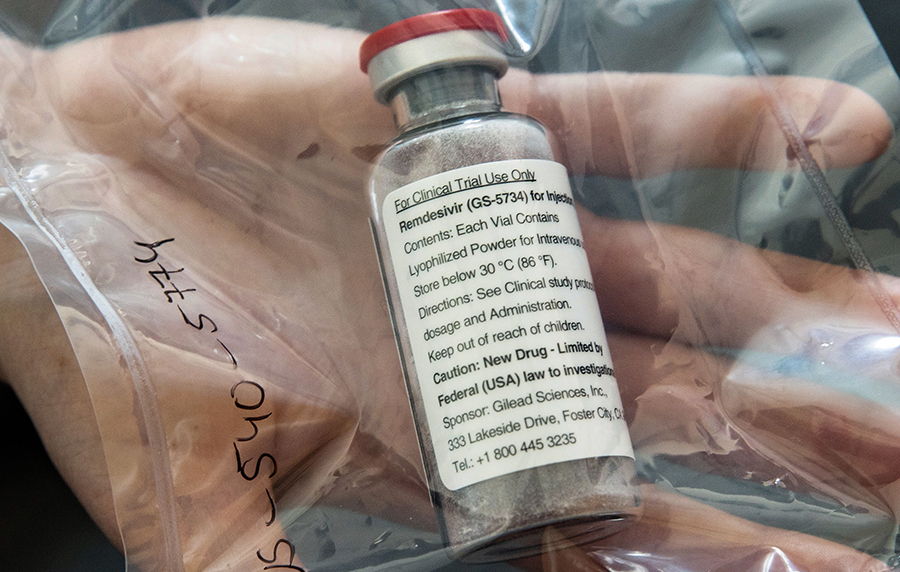
The US Food and Drug Administration (FDA) on Thursday granted full approval to Gilead Sciences Inc. for its antiviral drug, remdesivir, making it the first drug to obtain formal clearance for treating the coronavirus disease after conditional authorization was given in May.
The regulators had granted an emergency use authorization for remdesivir earlier this year, and since then, the drug has become a widely used therapy for hospitalized Covid-19 patients. It was also reportedly given to President Donald Trump this month, when he was diagnosed with the coronavirus disease.
READ: UK to roll out COVID-19 vaccine in less than 3 months and mass vaccination by Easter
This was disclosed in a statement by Gilead Sciences on Thursday.
In its statement, Gilead said, “Veklury is now the first and only approved Covid-19 treatment in the United States.’’ While the drug was in short supply initially, Gilead said that the medicine is now widely available in hospitals across the country as manufacturing capacity has rapidly expanded.
READ: COVID-19: US to have enough vaccines for vulnerable Americans by end of 2020
This is coming some days after the World Health Organization (WHO) study had discovered that the remdesivir anti-viral drug had little or no effect on Covid-19 patients’ length of stay in the hospital or chances of survival. The WHO said it failed to prevent deaths among patients.
However, Gilead has criticized the WHO study. In a letter posted on the company’s website, Chief Medical Officer Merdad Parsey said the findings didn’t negate other results.
READ: Remdesivir has received FDA’s emergency approval to treat COVID-19, according to Trump
The approval of remdesivir, sold under the brand name Veklury, will allow Gilead to market the drug and talk about its benefits to doctors, nurses, and patients. That could help solidify its position as a go-to medicine for Covid-19 patients, even as other drugs for the disease begin to reach the market.
Other treatments have received authorization for emergency use, although that approval can be revoked once the public health emergency caused by the coronavirus pandemic is over. Other medications like the steroid dexamethasone are also being used in the fight against Covid-19.
READ: COVID-19: World Bank approves $114 million response funds for Nigeria
Shares of Gilead gained 4.1% in trading after the close of regular market hours on Thursday. According to 13 analysts surveyed by Bloomberg, it is estimated that remdesivir will have sales of $2.17 billion this year.
The company said in June that it would charge U.S. hospitals roughly $3,120 for most patients who need remdesivir.
The approval is based on a U.S. government-sponsored trial involving over 1,000 hospitalized coronavirus patients that found that those who received the drug recovered about five days faster than those who got a placebo.
READ: Pfizer targets use of its Covid-19 vaccine by late November this year
The overall side-effect rate was similar to the placebo in the government study. The most common side effects are nausea and elevated liver enzymes, according to the product’s label.
What this means: This will be a very huge boost to the global search for a vaccine for the coronavirus pandemic which has negatively impacted on the global economy with over 1 million deaths. This also appears to be a boost for Donald Trump, who had sought the development of a Covid-19 vaccine before the US Presidential election.


















This information is misleading. A treatment or cure is different from a vaccine. Is Remdesivir a treatment or a vaccine because the title of this write-up does not correspond with the information provided. My thoughts
My observation too… treatment, not vaccine.
You guys suck.
Remdesivir isn’t a vaccine.