The Food and Drug Administration, on Wednesday, authorized booster shots of Pfizer and BioNTech’s Covid-19 for people who are 65 years and older and other vulnerable and immunocompromised citizens six months after they complete their first two doses.
This new move was as a result of the vaccine advisory committee recommendations on Friday, after more than an eight-hour agency meeting.
This decision still faces review by the Centers for Disease Control and prevention and its vaccine advisory committee is expected to vote this afternoon on the FDA’s proposal. Upon recommendation of approval, if the CDC endorses it, booster shots could commence immediately.
The FDA’s Vaccines Advisory Committee, last week, agreed unanimously to give boosters to older Americans and those who are highly vulnerable and are at high risk of suffering from severe illness should they contract the virus.
This came after a 16-2 vote against distributing the vaccines to Americans 16 and older.
The committee’s decision was informed by the vulnerability of people who fall under that classification as they are the most at risk of dying from Covid, accounting for more than 77% of all Covid deaths, according to the CDC, although these brackets make up roughly 17% of the U.S. population.
Emergency use authorization has been granted by the FDA for the administration of Pfizer’s shots to older Americans as well as to people aged 18 to 64 with medical conditions which makes them vulnerable and places them at risk of getting severely sick.
In addition, the agency included a broad definition of people from 18 to 64 “whose frequent institutional or occupational exposure” to the virus place them at a high risk of developing serious complications from Covid.
There’s a likelihood that the CDC would clear third doses for people working in nursing homes, prisons, front-line health employees and other essential workers who were amongst the first Americans to get the initial shots in December.
According to the agency’s top vaccine regulator, Dr. Peter Marks, “The FDA considered the committee’s input and conducted its own thorough review of the submitted data to reach today’s decision”.
“We will continue to analyze data submitted to the FDA pertaining to the use of booster doses of COVID-19 vaccines and we will make further decisions as appropriate based on the data.” he added.
Recall that, the Biden administration has said it wants to begin offering booster shots to the general public as early as this week, pending authorization from U.S. health regulators, hence the decision by the vaccine advisory committee is expected to be controversial.






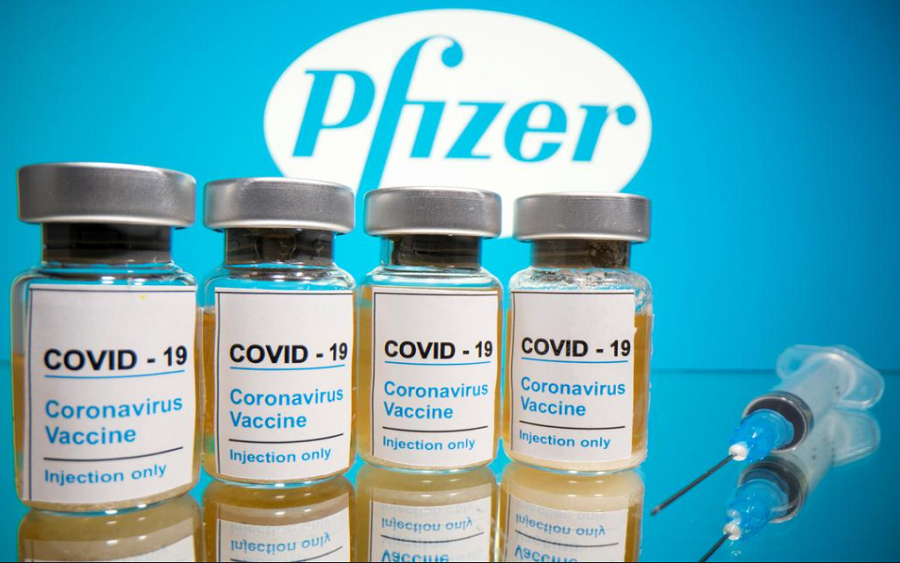












I didn’t get a corvic19 upto now