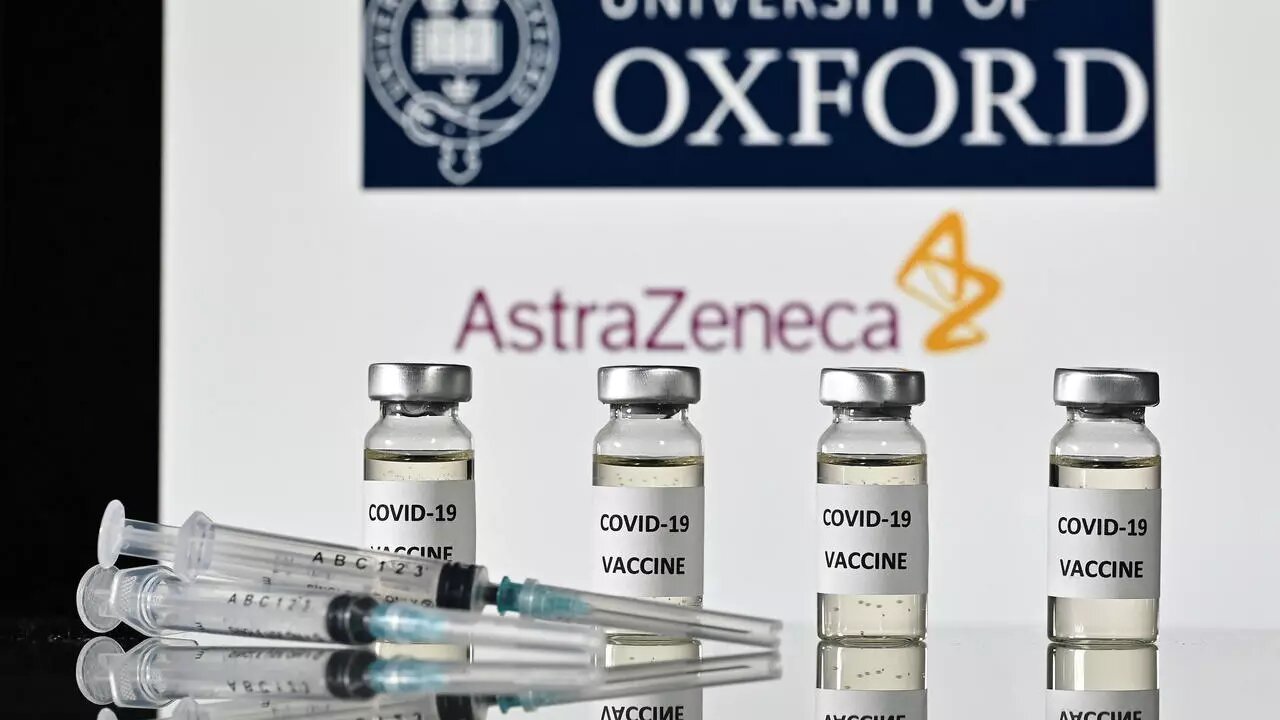The World Health Organization (WHO) has finally given approval for two versions of the AstraZeneca/Oxford Covid-19 vaccine for emergency use and to be rolled out globally via COVAX.
According to Dr Mariangela Simao, the WHO Assistant-director general for access to medicines and health products,
- “The vaccine can now be used via COVAX, a WHO-led international initiative for equitable access to Covid-19 vaccines.
- “Countries with no access to vaccines to date will finally be able to start vaccinating their health workers and populations at risk.
- “But we must keep up the pressure to meet the needs of priority populations everywhere and facilitate global access.”
What you should know
- The two versions are products of AstraZeneca-SKBio in South Korea and the Serum Institute of India.
- In a recent news release by WHO as reported by Xinhua news agency, the vaccine shows 63.09% efficacy and it is quite suitable for low- and middle-income countries due to easy storage requirements.
- According to Dr Mariangela Simao, two priorities are critical ie. a scale-up of manufacturing capacity, and developers’ early submission of their vaccines for the WHO’s review.
- The AstraZeneca product is the second Covid-19 jab to have received WHO authorisation, after Pfizer/BioNTech vaccine in late December 2020.