The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a public alert regarding the sale of falsified and unregistered brands of Oxytocin Injection in Nigeria.
These counterfeit products came to light during the Risk-Based Post-Marketing Surveillance (RBPMS) activities conducted by the Post-Marketing Surveillance Directorate of NAFDAC.
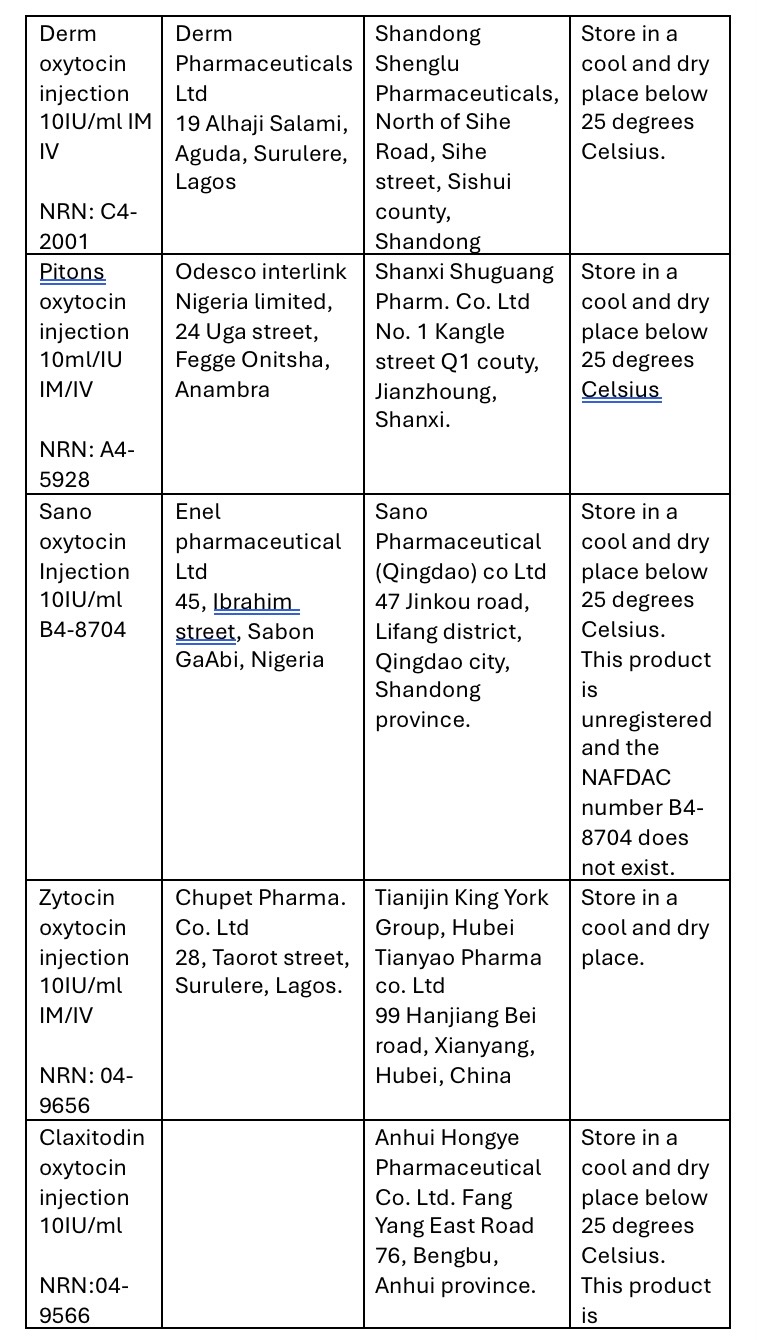
Upon initial physical screening, the products were found to be in gross violation of labelling requirements. The approved recommended storage temperature for Oxytocin Injection is between 2℃ to 8℃.
However, the discovered products stated a storage temperature of below 25℃, indicating a significant deviation from the approved conditions.
The falsified product details


What you should know
- Oxytocin injection is an essential drug used for inducing labour, controlling bleeding after childbirth, and treating incomplete or inevitable abortion.
- The illegal sale of unregistered or falsified medicines poses a substantial risk to public health.
- Failing to adhere to the recommended storage temperature compromises the safety, quality, and efficacy of the products.
- Administering falsified or unregistered oxytocin injections may cause harm to patients, leading to severe consequences, including death.
These counterfeit products have been distributed across various parts of the country through both legal and illegal channels. NAFDAC is taking swift action to detect and remove these products from circulation to prevent harm to patients. Consequently, the agency has instructed all State offices to conduct surveillance and recall all batches of these violating products.
NAFDAC urges importers, distributors, retailers, and healthcare providers to exercise caution and vigilance in the supply chain. All medicinal products should be obtained from authorized and licensed suppliers, with a thorough check of authenticity and physical condition.
Members of the public possessing the mentioned counterfeit products are advised to discontinue their sale or use and submit the stock to the nearest NAFDAC office.
If you come across these falsified or unregistered products, it is crucial not to use them. In case of any adverse reactions or events after using the product, immediate medical advice from a qualified healthcare professional is recommended.












